
1 RAGAGLITAZAR
2 TROGLITAZONE
3 MITOGLITAZONE
4 PIOGLITAZONE
5 NAVEGLITAZAR
6 SAROGLITAZAR
7 CIGLITAZONE
8
9
10
2 MURA
3 INDE
Indeglitazar



1
Ragaglitazar ........Dr. Reddy's Research Foundation
1
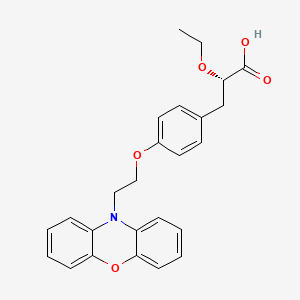
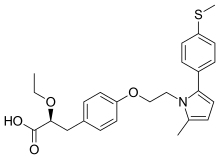
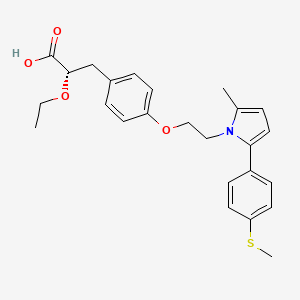
Ragaglitazar
NNC-61-0029, (-) – DRF-2725, NN-622,
(−)DRF 2725
cas 222834-30-2
222834-21-1 (racemate)
Hyperlipidemia; Hypertriglyceridemia; Lipid metabolism disorder; Non-insulin dependent diabetes
PPAR alpha agonist; PPAR gamma agonist
(2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY]PHENYL}PROPANOIC ACID,
(2S)-2-ethoxy-3-[4-(2-phenoxazin-10-ylethoxy)phenyl]propanoic acid, DRF, 1nyx
Molecular Formula: C25H25NO5
Molecular Weight: 419.4697 g/mol
Antidiabetic Drugs, ENDOCRINE DRUGS, Type 2 Diabetes Mellitus, Agents for, Insulin Sensitizers, PPARalpha Agonists, PPARgamma Agonists
Phase III
…………………..

EP 1049684; JP 2001519422; WO 9919313
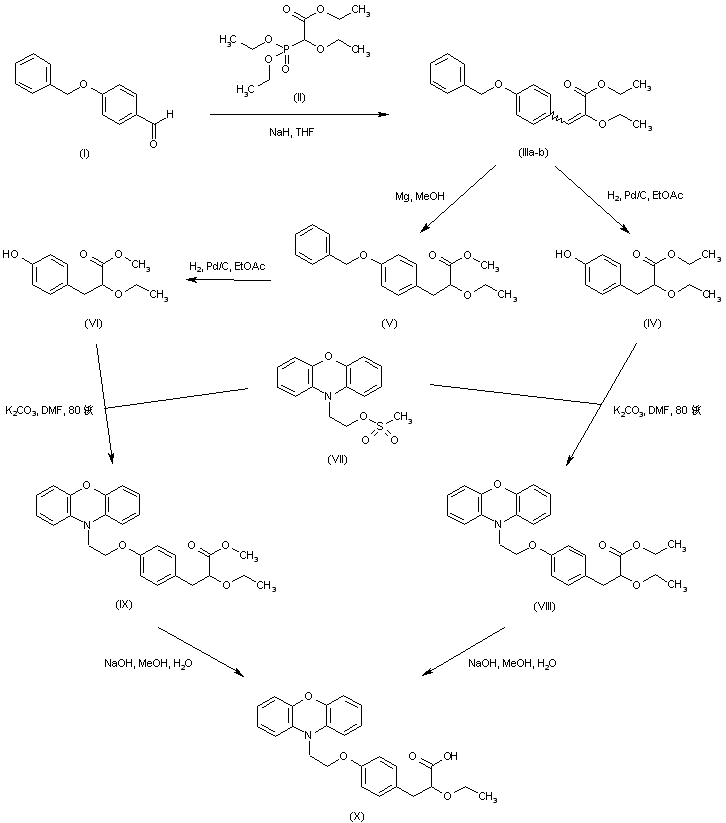
Several related procedures have been described for the synthesis of the title compound. The Horner-Emmons reaction of 4-benzyloxybenzaldehyde (I) with triethyl 2-ethoxyphosphonoacetate (II) afforded the unsaturated ester (IIIa-b) as a mixture of E/Z isomers. Simultaneous double-bond hydrogenation and benzyl group hydrogenolysis in the presence of Pd/C furnished phenol (IV). Alternatively, double-bond reduction by means of magnesium in MeOH was accompanied by transesterification, yielding the saturated methyl ester (V). Further benzyl group hydrogenolysis of (V) over Pd/C gave phenol (VI). The alkylation of phenols (IV) and (VI) with the phenoxazinylethyl mesylate (VII) provided the corresponding ethers (VIII) and (IX), respectively. The racemic carboxylic acid (X) was then obtained by hydrolysis of either ethyl- (VIII) or methyl- (IX) esters under basic conditions.

…………………………………………


……………………………………………………..
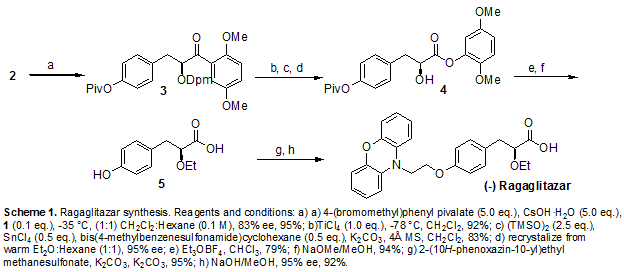
The synthesis of ragaglitazar (Scheme 1) was commenced by treating substrate 2 under optimized phase-transfer catalyzed conditions, using solid cesium hydroxide monohydrate as the base, a pivalate protected benzyl bromide and the Park and Jew triflurobenzyl-hydrocinchonidinium bromide salt 1. We were delighted to find that this reaction produced 3 in good yield with good selectivity. Subsequent removal of the diphenylmethyl (DPM) group under Lewis acidic conditions followed by a Baeyer-Villager like oxidation yielded the - hydroxy aryl ester 4. At this point, we were again pleased to find that this ester could be recrystalized from warm ether to give essentially enantiomerically pure products (~95% ee). The free hydroxyl was then alkylated using triethyloxonium tetrafluoroborate, and then transesterification under catalytic basic conditions produced 5. A mesylated phenoxazine alcohol reacted with 5 to yield the methyl ester of 6, which was obtained by treatment with sodium hydroxide in methanol. The overall synthesis proceeds with 47% overall yield (41% from commercially available reagents) and is eight linear steps from the alkoxyacetophenone substrate 2, including a recrystalization.

……………………………………………………………..

J Med Chem 2001,44(16),2675

(−)DRF 2725 (6) is a phenoxazine analogue of phenyl propanoic acid. Compound 6 showed interesting dual activation of PPARα and PPARγ. In insulin resistant db/db mice, 6 showed better reduction of plasma glucose and triglyceride levels as compared to rosiglitazone. Compound 6 has also shown good oral bioavailability and impressive pharmacokinetic characteristics. Our study indicates that 6 has great potential as a drug for diabetes and dyslipidemia.

Scheme 1 a
a (a) NaH, DMF, 0−25 °C, 12 h; (b) triethyl 2-ethoxy phosphosphonoacetate, NaH, THF, 0−25 °C, 12 h; (c) Mg/CH3OH, 25 °C, 12 h; (d) 10% aq NaOH, CH3OH, 25 °C, 6 h; (e) (1) pivaloyl chloride, Et3N, DCM, 0 °C, (2) (S)-2-phenyl glycinol/Et3N; (f) 1 M H2SO4, dioxane/water, 90−100 °C, 80 h.
Compound 6 is prepared from phenoxazine using a synthetic route shown in Scheme 1. Phenoxazine 7 upon reaction with p-bromoethoxy benzaldehyde 89 gave benzaldehyde derivative 9. Reacting 9 with triethyl 2-ethoxy phosphonoacetate afforded propenoate 10 as a mixture of geometric isomers. Reduction of 10 using magnesium methanol gave propanoate 11, which on hydrolysis using aqueous sodium hydroxide gave propanoic acid 12 in racemic form. Resolution of 12 using (S)(+)-2-phenyl glycinol followed by hydrolysis using sulfuric acid afforded the propanoic acid 6 in (−) form.
Nate, H.; Matsuki, K.; Tsunashima, A.; Ohtsuka, H.; Sekine, Y. Synthesis of 2-phenylthiazolidine derivatives as cardiotonic agents. II. 2-(phenylpiperazinoalkoxyphenyl)thiazolidine-3-thiocarboxyamides and corresponding carboxamides. Chem. Pharm. Bull. 1987, 35, 2394−2411
(S)-3-[4-[2-(Phenoxazin-10-yl)ethoxy]phenyl]-2-eth-oxypropanoic Acid (6). as a white solid, mp: 89−90 °C.
[α]D 25 = − 12.6 (c = 1.0%, CHCl3).
1H NMR (CDCl3, 200 MHz): δ 1.16 (t, J = 7.0 Hz, 3H), 1.42−1.91 (bs, 1H, D2O exchangeable), 2.94−3.15 (m, 2H), 3.40−3.65 (m, 2H), 3.86−4.06 (m, 3H), 4.15 (t, J = 6.6 Hz, 2H), 6.63−6.83 (m, 10H), 7.13 (d, J = 8.5 Hz, 2H). Mass m/z (relative intensity): 419 (M+, 41), 197 (15), 196 (100), 182 (35), 167 (7), 127 (6), 107 (19).
Purity by HPLC: chemical purity: 99.5%; chiral purity: 94.6% (RT 27.5).

…………………………………

EXAMPLE 23 (−) 3-[4-[2-(phenoxazin-10-yl)ethoxy]phenyl]-2-ethoxypropanoic acid:
The title compound (0.19 g, 54%) was prepared as a white solid from diastereomer [(2S-N(1S)]-3-[4-[2-(phenoxazin-10-yl)ethoxy]phenyl]-2-ethoxy-N-(2-hydroxy-1-phenyl)ethylpropanamide (0.45 g, 0.84 mmol) obtained in example 21b by an analogous procedure to that described in example 22. mp: 89-90° C.
[α]D 25=−12.6 (c=1.0% CHCl3)
1H NMR (CDCl3, 200 MHz): δ 1.16 (t, J=7.02 Hz, 3H), 1.42-1.91 (bs, 1H, D2O exchangeable), 2.94-3.15 (complex, 2H), 3.40-3.65 (complex, 2H), 3.86-4.06 (complex, 3H), 4.15 (t, J=6.65 Hz, 2H), 6.63-6.83 (complex, 10H), 7.13 (d, J=8.54 Hz, 2H).
………………………..
Example 23
(S)-3-[4-[2-(phenoxazin-10-yl)ethoxy]phenyl]-2-ethoxypropanoic acid :
The title compound (0.19 g, 54 %) was prepared as a white solid from diastereomer [(2S- N(lS)]-3-[4-[2-(phenoxazin-10-yl)ethoxy]phenyl]-2-ethoxy-N-(2-hydroxy-l- phenyl)propanamide (0.45 g, 0.84 mmol) obtained in example 21b by an analogous procedure to that described in example 22. mp : 89 – 90 °C. [α]D 25 = – 12.6 (c = 1.0 %, CHC13)
*H NMR (CDC13, 200 MHz) : δ 1.16 (t, J = 7.02 Hz, 3H), 1.42 – 1.91 (bs, IH, D20 exchangeable), 2.94 – 3.15 (complex, 2H), 3.40 – 3.65 (complex, 2H), 3.86 – 4.06 (complex, 3H), 4.15 (t, J = 6.65 Hz, 2H), 6.63 – 6.83 (complex, 10H), 7.13 (d, J = 8.54 Hz, 2H).

| PATENT | SUBMITTED | GRANTED |
|---|---|---|
| Benzamides as ppar modulators [US2006160894] | 2006-07-20 | |
| Novel tricyclic compounds and their use in medicine process for their preparation and pharmaceutical compositions containing them [US2002077320] | 2002-06-20 | |
| Tricyclic compounds and their use in medicine process for their preparation and pharmaceutical compositions containing them [US7119198] | 2006-07-06 | 2006-10-10 |
| Tricyclic compounds and their use in medicine: process for their preparation and pharmaceutical compositions containing them [US6440961] | 2002-08-27 | |
| Tricyclic compounds and their use in medicine process for their preparation and pharmaceutical compositions containing them [US6548666] | 2003-04-15 | |
| Tricyclic compounds and their use in medicine process for their preparation and pharmaceutical compositions containing them [US6608194] | 2003-08-19 | |
| CRYSTALLINE R- GUANIDINES, ARGININE OR (L) -ARGININE (2S) -2- ETHOXY -3-{4- [2-(10H -PHENOXAZIN -10-YL)ETHOXY]PHENYL}PROPANOATE [WO0063189] | 2000-10-26 | |
| Pharmaceutically acceptable salts of phenoxazine and phenothiazine compounds [US6897199] | 2002-11-14 | 2005-05-24 |
| Tricyclic compounds and their use in medicine process for their preparation and pharmaceutical compositions containing them [US6939988] | 2005-09-06 |

WO-2014181362
wo/2014/181362 a process for the preparation of 3 … – WIPO
patentscope.wipo.int/search/en/WO2014181362
Nov 13, 2014 - (WO2014181362) A PROCESS FOR THE PREPARATION OF 3-ARYL-2-HYDROXY PROPANOIC ACID COMPOUNDS …
A process for the preparation of 3-aryl-2-hydroxy propanoic acid compounds
ragaglitazar; saroglitazar
Council of Scientific and Industrial Research (India)
Process for preparing enantiomerically pure 3-aryl-2-hydroxy propanoic acid derivatives (eg ethyl-(S)-2-ethoxy-3-(4-hydroxyphenyl)propanoate), using S-benzyl glycidyl ether as a starting material. Useful as intermediates in the synthesis of peroxisome proliferator activated receptor agonist such as glitazars (eg ragaglitazar or saroglitazar). Appears to be the first filing on these derivatives by the inventors; however see WO2014181359 (for a concurrently published filing) and US8748660 (for a prior filing), claiming synthesis of enantiomerically pure compounds.
- Dolling, U. H.; Davis, P.; Grabowski, E. J. J. Efficient Catalytic Asymmetric Alkylations. 1. Enantioselective Synthesis of (+)-Indacrinone via Chiral Phase-Transfer Catalysis. J. Am. Chem. Soc. 1984, 106, 446–447.
- Andrus, M. B.; Hicken, E. J.; Stephens, J. C. Phase-Transfer Catalyzed Asymmetric Glycolate Alkylation. Org. Lett. 2004, 6, 2289–2292.
- Andrus, M. B.; Hicken, E. J.; Stephens, J. C.; Bedke, D. K. Asymmetric Phase-Transfer Catalyzed Glycolate Alkylation, Investigation of the Scope, and Application to the Synthesis of (-)-Ragaglitazar. J. Org. Chem. 2005, ASAP.
- Henke, B. R. Peroxisome Proliferator-Activated Receptor Dual Agonists for the Treatment of Type 2 Diabetes. J. Med. Chem. 2004, 47, 4118–4127.
- Wilson, T. M.; Brown, P. J.; Sternbach, D. D.; Henke, B. R. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 2000, 46, 1306–1317.
- Uchida, R.; Shiomi, K.; Inokoshi, J.; Masuma, R.; Kawakubo, T.; Tanaka, H.; Iwai, Y.; Omura, A. Kurasoins A and B, New Protein Farnesyltrasferase Inhibitors Produced by Paecilomyces sp. FO-3684. J. Antibio. 1996, 49, 932–934.


.......................
2
TROGLITAZONE

Troglitazone, GR-92132X, CI-991, CS-045, Romozin, Prelay, Rezulin, Noscal
CAS 97322-87-7
C24 H27 N O5 S, 441.54
(±)-5-[4-(6-Hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran-2-ylmethoxy)benzyl]thiazolidine-2,4-dione
2,4-Thiazolidinedione, 5-[[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)methoxy]phenyl]methyl]-
- CI 991
- CS 045
- Depotox
- GR 92132X
- Noscal
- Rezulin
- Romglizone
- Troglitazone
Withdrawn – 2000
Crystals, m.p. 184-6 °C
Daiichi Sankyo Co., Ltd. INNOVATOR
Trademarks: Noscal (Sankyo); Prelay (Sankyo); Rezulin
Percent Composition: C 65.28%, H 6.16%, N 3.17%, O 18.12%, S 7.26%
Properties: Crystals from benzene-acetone, mp 184-186°.
Therap-Cat: Antidiabetic.
Insulin Sensitizer.
Troglitazone
 Type-II diabetes mellitus (DM) is characterized by insulin resistance, glucose intolerance, increased hepatic glucose production, and decreased pancreatic insulin secretion. In the past, the drug classes used for type-II DM have targeted the last three of these abnormalities. Sulfonylurea agents bind to ATP-dependent potassium efflux channels to stimulate pancreatic insulin secretion at b-islet cells. The biguanides decrease hepatic glucose production, and thea-glucosidase inhibitors delay carbohydrate digestion to improve glucose tolerance. Until the recent advent of the thiazolidinedione drugs (ciglitazone was first synthesized in 1982), there was no therapy specifically targeting insulin resistance. Drugs of this class all share a common thiazolidine-2-4-dione structure. Marketed drugs of this class include pioglitazone, rosiglitazone, and troglitazone [Figure 1] – the first to reach the market.
Type-II diabetes mellitus (DM) is characterized by insulin resistance, glucose intolerance, increased hepatic glucose production, and decreased pancreatic insulin secretion. In the past, the drug classes used for type-II DM have targeted the last three of these abnormalities. Sulfonylurea agents bind to ATP-dependent potassium efflux channels to stimulate pancreatic insulin secretion at b-islet cells. The biguanides decrease hepatic glucose production, and thea-glucosidase inhibitors delay carbohydrate digestion to improve glucose tolerance. Until the recent advent of the thiazolidinedione drugs (ciglitazone was first synthesized in 1982), there was no therapy specifically targeting insulin resistance. Drugs of this class all share a common thiazolidine-2-4-dione structure. Marketed drugs of this class include pioglitazone, rosiglitazone, and troglitazone [Figure 1] – the first to reach the market.
The “glitazones” act to reduce insulin resistance and also correct hyperglycemia, hyperinsulinemia, and hypertriglyceridemia. Thiazolidinediones bind to the gisoform of the peroxisome proliferator-activated receptor (PPARg), a nuclear transcription factor that regulates the expression of several insulin-responsive genes involved in glucose and lipid metabolism, and the differentiation of fibroblasts into adipose tissue. The net effect is to reduce insulin resistance, mostly through increased glucose uptake by muscle tissue; however, the exact biochemical mechanism is unclear. Effects on lipid metabolism include decreased triglycerides and free fatty acids, and a slight increase or no change in high-density lipoprotein, low-density lipoprotein, and total cholesterol. There also appear to be acute increases in insulin-stimulated glucose uptake that are PPAR-independent. This effect is too rapid to occur via gene transcription, and in the case of troglitazone may result from action of its quinone metabolite. Troglitazone also decreases production of various inflammatory mediators and may antagonize TNFa.
Troglitazone�s most common adverse effect is fluid retention, which may increase preload and induce cardiac hypertrophy. Troglitazone is contraindicated in congestive heart failure, and cases of pulmonary edema have been reported. Troglitazone induces colon polyps in murine models and is therefore contraindicated for patients with familial polyposis coli. Troglitazone and pioglitazone (but not rosiglitazone) induce cytochrome P450 (CYP) 3A4. This enzyme induction can result in decreased drug levels or drug effects with estradiol, terfenadine, cyclosporine, simvistatin, tacrolimus, and other drugs metabolized by CYP 3A4. A small fraction of troglitazone is metabolized by CYP (not 3A4) to an active quinone metabolite, but it is mostly conjugated to sulfate and glucuronide. Troglitazone enhances the anticoagulant effect of warfarin, probably through competitive serum protein binding, and has other drug interactions at the PPAR level. Troglitazone interferes with gemfibrozil’s binding to PPARa, and may decrease NSAID effectiveness by competing for PPARg.
Rezulin� (tradename troglitazone by Parke-Davis) was FDA approved January 29, 1997, and was first marketed in March 1997. Over 600,000 American patients received troglitazone, with an additional 200,000 in Japan. Pre-marketing studies showed 1.9% of patients on troglitazone developed serum alanine aminotransferase levels in excess of three times the upper limit of normal, vs. 0.6% with placebo. Such hepatotoxicity was typically asymptomatic and reversible. A few patients developed overt liver injury, and two liver biopsies among these patients showed hepatocellular injury pattern. It was estimated that only 1 patient in 50,000 to 60,000 would die from liver failure or require liver transplantation. On November 3, 1997, the FDA released a warning regarding 150 adverse events with troglitazone, 35 with acute liver injury, and 3 deaths in Japan from liver failure. The warnings and restrictions about troglitazone were extended in December 1997, July 1998, and June 1999. Troglitazone was voluntarily withdrawn from the US market on March 21, 2000, after it had been demonstrated that Rezulin� was more toxic than either Avandia� (rosiglitazone) or Actos�(pioglitazone).
Troglitazone hepatotoxicity appears to be idiosyncratic. The onset is typically delayed, usually 2-5 months after initiating therapy, although one case was reported after only four doses. Although hypersensitivity has been suggested in several cases, the hallmarks of immune mechanisms, fever, rash, and eosinophilia, are usually absent. Histologic specimens usually show hepatocellular injury, bridging fibrosis and necrosis, intracanalicular cholestasis, and lack of regenerative activity. Samples vary in the amount of WBC infiltration (with or without eosinophils) and steatosis.
Idiosyncratic (or host-dependent) drug reactions are either due to hypersensitivity or to metabolic aberrations. It is not clear whether troglitazone hepatotoxicity is caused by hypersensitivity. Proposed metabolic aberrations include oxidation/reduction reactions with thea-tocopherol moiety on troglitazone (although it is usually considered an antioxidant), reactions from the quinone metabolite (similar to acetaminophen’s quinone metabolite), and genetic variations in cytokines and their receptors, the apoptosis cascade, mitochondrial respiration, and regenerative response. It is unlikely that CYP polymorphisms play a major role, as the incidence of troglitazone hepatotoxicity is too low. Two cases of hepatic toxicity associated with rosiglitazone have also been reported. Although the patients had co-morbidities, exposures to other drugs, and one case may have been due to shock, these cases suggest that hepatotoxicity may be an emerging “class-effect” of thiazolidinediones.
Troglitazone (Rezulin, Resulin, Romozin, Noscal) is an antidiabetic and anti-inflammatory drug, and a member of the drug class of thethiazolidinediones. It was prescribed for patients with diabetes mellitus type 2.[1] It was developed by Daiichi Sankyo Co.(Japan). In the United States, it was introduced and manufactured by Parke-Davis in the late 1990s, but turned out to be associated with anidiosyncratic reaction leading to drug-induced hepatitis. One F.D.A. medical officer evaluating troglitazone, John Gueriguian, did not recommend its approval due to potential high liver toxicity,[2] but a full panel of experts approved it in January 1997.[3] Once the prevalence of adverse liver effects became known, troglitazone was withdrawn from the British market in December 1997, from theUnited States market in 2000, and from the Japanese market soon afterwards. It didn’t get approval in the rest of Europe.
Approval history
Troglitazone was developed as the first anti-diabetic drug having a mechanism of action involving the enhancement of insulin sensitivity. At the time it was widely believed that such drugs, by addressing the primary metabolic defect associated with Type 2 diabetes, would have numerous benefits including avoiding the risk of hypoglycemia associated with insulin and earlier oral antidiabetic drugs. It was further believed that reducing insulin resistance would potentially reduce the very high rate of cardiovascular disease that is associated with diabetes.[4][5]
Parke-Davis/Warner Lambert submitted the diabetes drug Rezulin for U.S. Food and Drug Administration (F.D.A.) review on July 31, 1996. The medical officer assigned to the review, Dr. John L. Gueriguian, cited Rezulin’s potential to harm the liver and the heart and he questioned its viability in lowering blood sugar for patients with adult-onset diabetes, recommending against the drug’s approval. After complaints from the drugmaker, Gueriguian was removed on November 4, 1996 and his review was purged by the F.D.A.[6][7]Gueriguian and the company had a single meeting, at which Gueriguian used “intemperate” language; The company said it’s objections were based on inappropriate remarks made by Gueriguian.[8] Parke-Davis said at the advisory committee that the risk of liver toxicity was comparable to placebo and that additional data of other studies confirmed this.[9] According to Peter Gøtzsche, when the company provided these additional data one week after approval, they showed a substantial greater risk for liver toxicity.[10]
The F.D.A. approved the drug on January 29, 1997, and it appeared in pharmacies in late March. At the time Dr. Solomon Sobel, a director at the F.D.A., overseeing diabetes drugs, said in a New York Times interview that adverse effects of troglitazone appeared to be rare and relatively mild.[11]
Glaxo Wellcome P.L.C. received approval from the British Medicines Control Agency (MCA) to market troglitazone, as Romozin, in July 1997.[12] After reports of sudden liver failure in patients receiving the drug, the Parke-Davis and the FDA added warnings to the drug label requiring monthly monitoring of liver enzyme levels.[13] Glaxo removed troglitazone from the market in Britain on December 1, 1997.[6] Glaxo had licensed the drug from Sankyo Company of Japan and had sold it in Britain from October 1, 1997.[14][15]
On May 17, 1998, a 55-year old patient named Audrey LaRue Jones died of acute liver failure after taking troglitazone. Importantly, she had been monitored closely by physicians at the National Institutes of Health as a participant in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) diabetes prevention study.[16][17] This called into question the efficacy of the monitoring strategy. The N.I.H. responded on June 4 by dropping troglitazone from the study.[7][18] Dr. David J. Graham, an F.D.A. epidemiologist charged with evaluating the drug, warned on March 26, 1999 of the dangers of using it and concluded that patient monitoring was not effective in protecting against liver failure. He estimated that the drug could be linked to over 430 liver failures and that patients incurred 1,200 times greater risk of liver failure when taking Rezulin.[7][19] Dr. Janet B. McGill, an endocrinologist who had assisted in the Warner–Lambert’s early clinical testing of Rezulin, wrote in a March 1, 2000 letter to Sen. Edward M. Kennedy (D-Mass.): “I believe that the company . . . deliberately omitted reports of liver toxicity and misrepresented serious adverse events experienced by patients in their clinical studies.”[20]
On March 21, 2000, the F.D.A. withdrew the drug from the market.[21] Dr. Robert I. Misbin, an F.D.A. medical officer, wrote in a July 3, 2000 letter to the House Energy and Commerce Committee of strong evidence that Rezulin could not be used safely, after having been threatened by the FDA with dismissal in March 2000.[6][22] By that time the drug had been linked to 63 liver-failure deaths and had generated sales of more than $2.1 billion for Warner-Lambert.[19] The drug cost $1,400 a year per patient in 1998.[15] Pfizer, which had acquired Warner-Lambert in February 2000, reported the withdrawal of Rezulin cost $136 million.[23]
Lawsuits
In 2009 Pfizer Inc. resolved all but three of 35,000 claims over its withdrawn diabetes drug Rezulin for a total of about $750 million. Pfizer, which acquired rival Wyeth for almost $64 billion, paid about $500 million to settle Rezulin cases consolidated in federal court in New York, according to court filings. The company also paid as much as $250 million to resolve state-court suits. In 2004, it set aside $955 million to end Rezulin cases.[24]
Mode of action
Troglitazone, like the other thiazolidinediones (pioglitazone and rosiglitazone), works by activating peroxisome proliferator-activated receptors (PPARs).
Troglitazone is a ligand to both PPARα and – more strongly – PPARγ. Troglitazone also contains an α-tocopheroyl moiety, potentially giving it vitamin E-like activity in addition to its PPAR activation. It has been shown to reduce inflammation:[25] troglitazone use was associated with a decrease of nuclear factor kappa-B (NF-κB) and a concomitant increase in its inhibitor (IκB). NFκB is an important cellular transcription regulator for the immune response.


rosiglitazone, ciglitazone, darglitazone, englitazone, rosiglitazone, pioglitazone, rosiglitazone, troglitazone
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| (RS)-5-(4-[(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy]benzyl)thiazolidine-2,4-dione | |
| CLINICAL DATA | |
| LEGAL STATUS |
?
|
| PHARMACOKINETIC DATA | |
| HALF-LIFE | 16-34 hours |
| IDENTIFIERS | |
| CAS NUMBER | 97322-87-7 |
| ATC CODE | A10BG01 |
| PUBCHEM | CID 5591 |
| IUPHAR LIGAND | 2693 |
| DRUGBANK | DB00197 |
| CHEMSPIDER | 5389 |
| UNII | I66ZZ0ZN0E |
| KEGG | D00395 |
| CHEBI | CHEBI:9753 |
| CHEMBL | CHEMBL408 |
| CHEMICAL DATA | |
| FORMULA | C24H27NO5S |
| MOL. MASS | 441.541 g/mol |
………………….

………………..

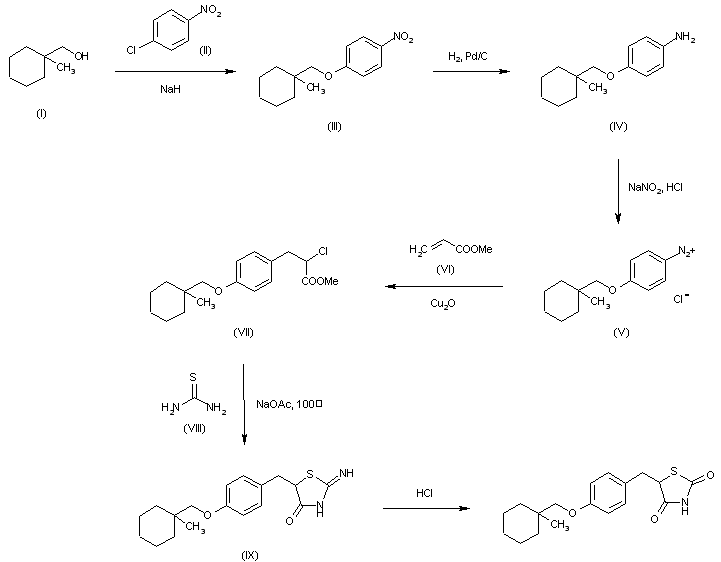
A new synthesis of [14C]-labeled CS-045 has been reported: The condensation of 5-acetoxy-2-hydroxy-3,4,6-trimethylacetophenone (I) with phenoxyacetone (II) by means of morpholine and p-toluenesulfonic acid in refluxing benzene gives 6-acetoxy-2,5,7,8-tetramethyl-2-(phenoxymethyl)-3,4-dihydro-2H-benzo[b]pyran-4-one (III), which is reduced with NaBH4 in methanol to the corresponding carbinol (IV). The dehydration of (IV) by means of p-toluenesulfonic acid in refluxing benzene affords 2-acetoxy-2,5,7,8-tetramethyl-2-(phenoxymethyl)-2H-benzo[b]pyran (V), which is hydrogenated with H2 over Pd/C in methanol to give the corresponding 3,4-dihydro derivative (VI). The hydrolysis of (VI) with NaOH in methanol yields the corresponding phenol (VII), which is chloromethylated with paraformaldehyde and dry HCl in dioxane to afford 2-[4-(chloromethyl)phenoxymethyl]-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[b]pyran-6-ol (VIII). The protection of (VIII) with chloromethyl methyl ether by means of potassium tert-butoxide in THF gives the corresponding 6-(methoxymethoxy) derivative (IX), which is condensed with [5-14C]-thiazolidine-2,4-dione (X) by means of butyllithium in THF-HMPT to yield 5-[4-[6-(methoxymethoxy)-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[b]pyran-2-ylmethoxy]benzyl]-[5-14C]-thiazolidine-2,4-dione (XI). Finally, this compound is deprotected with concentrated HCl in ethylene glycol monomethyl ether at 130 C.

……………….

A new short synthesis of troglitazone has been described: Condensation of the bromoacetal (I) with 4-hydroxybenzaldehyde (II) by means of K2CO3 and NaI in refluxing acetone gives the unsaturated ether (III), which is cyclized with trimethylhydroquinone (IV) by means of bis(trifluoromethylsulfonyl)imide in dichloromethane to yield the 6-hydroxybenzopyran (V). Acylation of (V) with acetic anhydride and DMAP in THF affords the expected acetoxybenzopyran (VI), which is condensed with thiazolidine-2,4-dione (VII) by means of piperidine in toluene to provide the 6-benzylidene-thiazolidine (VIII). The hydrogenation of (VIII) with H2 over Pd/C in methanol gives the corresponding benzyl derivative (IX), which is finally deacetylated with AcOH/HCl/water (3:1:1) in MeOH.

…………..
European Journal of Medicinal Chemistry, 51, 206-215; 2012

……………………………………….
see Indian Journal of Heterocyclic Chemistry, 15(4), 407-408; 2006
……………………………………………………….
Bioorganic & Medicinal Chemistry Letters, 14(10), 2547-2550; 2004
Figure 1.
Scheme 2.
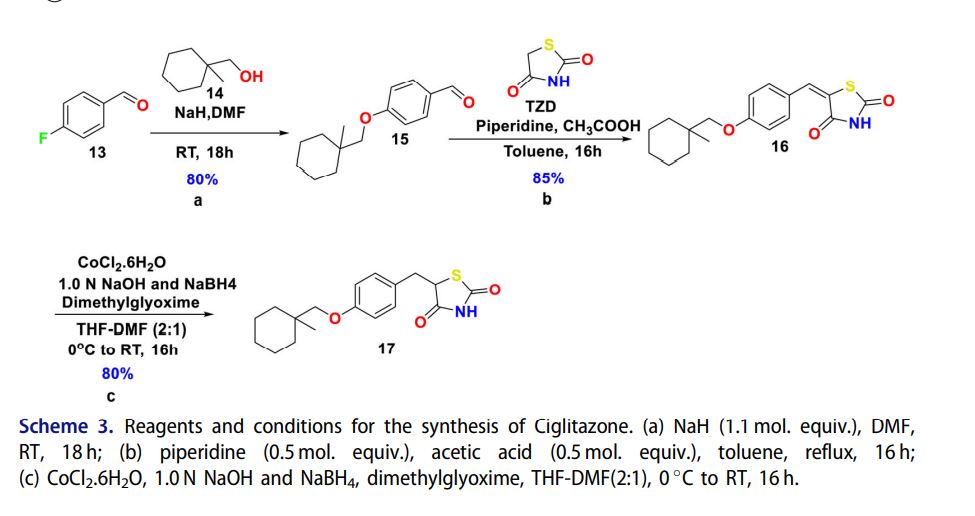
(a) t-Butyldimethylsilyl chloride, imidazole, DMF; (b) LAH, rt, 3 h (75.9%, two steps); (c) 4-fluorobenzaldehyde, KtOBu, DMF, 80 °C, 8 h; (d) 2,4-thiazolidinedione, AcOH, piperidine, toluene, reflux, 4 h (37%, two steps); (e) HCl, MeOH, 15 min; (f) CoCl2, DMG (84%).
………………………
Patent
EXAMPLE-1
A mixture of 70 g of ethyl-3- 4-(6-acetoxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)phenyl!-2-chloropropionate, 13.12 g of thiourea and 80.2 ml of sulpholane was reacted for 80 min., under a nitrogen stream at 115°-120° C. Subsequently, a 656.2 ml Acetic acid, 218.7 ml conc. hydrochloric acid and 109.4 ml water was added to this and the resulting mixture was further heated for 12 hrs at 85°-90° C. The reaction mixture was cooled to room temperature and 196.8 g of sodium bicarbonate was added and once the evolution of carbondioxide had ceased, the solvent was distilled off applying high vacuum. A 10:1 by volume mixture of benzene and ethyl acetate was added to the residue and the crude product was washed with a mixture of equal volumes of a saturated aq. sodium bicarbonate solution & water. The organic layer was dried over anhydrous sodium sulphate and the solvent was distilled off. The resulting crude product was quickly eluted from a silica gel column with 50% ethylacetate-hexane to furnish 40 g of the required 5-{4-(6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-yl-methoxy) benzyl) thiazolidine-2,4-dione (Troglitazone) with a HPLC purity of ˜67-70%. The elution of column was continued further to yield 5- 4-(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl-methoxy)benzyl!2-iminothiazolidine-4-one with HPLC purity of ˜70%.
Lit References:
Oral hypoglycemic agent which improves insulin sensitivity and decreases hepatic glucose production. Prepn: JP Kokai 85 51189; T. Yoshioka et al., US 4572912 (1985, 1986 both to Sankyo); T. Yoshioka et al.,
J. Med. Chem. 32,421 (1989).
Mechanism of action studies: T. P. Ciaraldi et al., Metabolism 39, 1056 (1990); M. Kellerer et al., Diabetes 43, 447 (1994).
Clinical evaluation: T. Kuzuya et al., Diabetes Res. Clin. Pract. 11, 147 (1991).
Clinical metabolic effects: S. L. Suter et al., Diabetes Care 15, 193 (1992).
References
- Fisher, Lawrence (4 November 1997). “Adverse Diabetes Drug News Sends Warner-Lambert Down”. The New York Times. Retrieved 12 December 2012.
- Retired Drugs: Failed Blockbusters, Homicidal Tampering, Fatal Oversights, wired.com
- Cohen, J. S. (2006). “Risks of troglitazone apparent before approval in USA”.Diabetologia 49 (6): 1454–5. doi:10.1007/s00125-006-0245-0. PMID 16601971.
- Henry RR (September 1996). “Effects of troglitazone on insulin sensitivity”. Diabet. Med.13 (9 Suppl 6): S148–50.PMID 8894499.
- Keen H (November 1994). “Insulin resistance and the prevention of diabetes mellitus”. N. Engl. J. Med. 331 (18): 1226–7.doi:10.1056/NEJM199411033311812. PMID 7935664.
- Willman, David (20 December 2000). “NEW FDA: Rezulin Fast-Track Approval and a Slow Withdrawal”. The Los Angeles Times. Retrieved 12 December 2012.
- Willman, David (4 June 2000). “The Rise and Fall of the Killer Drug Rezulin”. The Los Angeles Times. Retrieved 12 December 2012.
- “Report: FDA Removes Medical Officer”.
- Avorn, J (2005). Powerful medicines. New York: Vintage books.
- Gøtzsche, Peter (2013). Deadly medicines and organised crime : how big pharma has corrupted healthcare. London [u.a.]: Radcliffe Publ. p. 185. ISBN 9781846198847.
- Leary, Warren (31 January 1997). “New Class of Diabetes Drug Is Approved”. The New York Times. Retrieved 12 December 2012.
- Sinclair, Neil (31 July 1997). “Glaxo Wellcome gets approval for Romozin”. ICIS News. Retrieved 12 December 2012.
- “www.accessdata.fda.gov”.
- British Broadcasting Corporation (1 December 1997). “Diabetes drug withdrawn from sale”. BBC. Retrieved 12 December 2012.
- Fisher, Lawrence (17 January 1998). “Drug Makers at Threshold of a New Therapy; With a Dose of Biotechnology, Big Change Is Ahead in the Treatment of Diabetes”. The New York Times. Retrieved 12 December 2012.
- Diabetes Prevention Research Group (April 1999). “Design and methods for a clinical trial in the prevention of type 2 diabetes”.Diabetes Care 22 (4): 623–634.doi:10.2337/diacare.22.4.623. Retrieved 12 December 2012.
- Diabetes Prevention Program Research Group (7 February 2002). “Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin”. The New England Journal of Medicine 346 (6): 393–403.doi:10.1056/NEJMoa012512.PMC 1370926. PMID 11832527. Retrieved 12 December 2012.
- Gale, Edwin (January 2006). “Troglitazone: the lesson that nobody learned?”.Diabetologia 49 (1): 1–6. doi:10.1007/s00125-005-0074-6.
- Willman, David (16 August 2000). “FDA’s Approval and Delay in Withdrawing Rezulin Probed”. The Los Angeles Times. Retrieved 12 December 2012.
- Willman, David (10 March 2000). “Fears Grow Over Delay in Removing Rezulin”. The Los Angeles Times. Retrieved 12 December 2012.
- U.S. Food and Drug Administration. “2000 Safety Alerts for Human Medical Products”. U.S. Food and Drug Administration. Retrieved 12 December 2012.
- Willman, David (March 17, 2000). “Physician Who Opposes Rezulin Is Threatened by FDA With Dismissal”. Los Angeles Times.
- Pfizer. “Pfizer Annual Report 2001″. Pfizer. Retrieved 12 December 2012.
- Feeley, Jef (March 31, 2009). “Pfizer Ends Rezulin Cases With $205 Million to Spare”.Bloomberg. Retrieved 6 April 2014.
- Aljada A, Garg R, Ghanim H, et al. (2001). “Nuclear factor-kappaB suppressive and inhibitor-kappaB stimulatory effects of troglitazone in obese patients with type 2 diabetes: evidence of an antiinflammatory action?”. J. Clin. Endocrinol. Metab. 86 (7): 3250–6.doi:10.1210/jc.86.7.3250. PMID 11443197.
External links
- Diabetes Monitor article on troglit
| US4316849 * | 11 Jul 1980 | 23 Feb 1982 | Blasinachim S.P.A. | Process for preparing a crystalline polymorphous type of chenodeoxycholic acid |
| US4572912 * | 28 Aug 1984 | 25 Feb 1986 | Sankyo Company Limited | Treatment of hyperlipemia and hyperglycemia |
| US5248699 * | 13 Aug 1992 | 28 Sep 1993 | Pfizer Inc. | Hydrochloride salt, antidepressant, anorectic |
| US5319097 * | 11 Dec 1991 | 7 Jun 1994 | Imperial Chemical Industries Plc | Pharmaceutical agents |
| AU3255984A* | Title not available | |||
| EP0014590A1* | 7 Feb 1980 | 20 Aug 1980 | Eli Lilly And Company | Crystalline forms of N-2-(6-methoxy)benzothiazolyl-N’-phenyl urea and process for their preparation |
| EP0022527A1* | 4 Jul 1980 | 21 Jan 1981 | BLASCHIM S.p.A. | Process for preparing a solvent-free crystalline polymorphous form of chenodeoxycholic acid |
| EP0490648A1* | 11 Dec 1991 | 17 Jun 1992 | Zeneca Limited | Pharmaceutical agents |





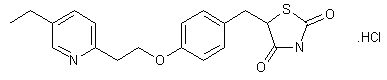
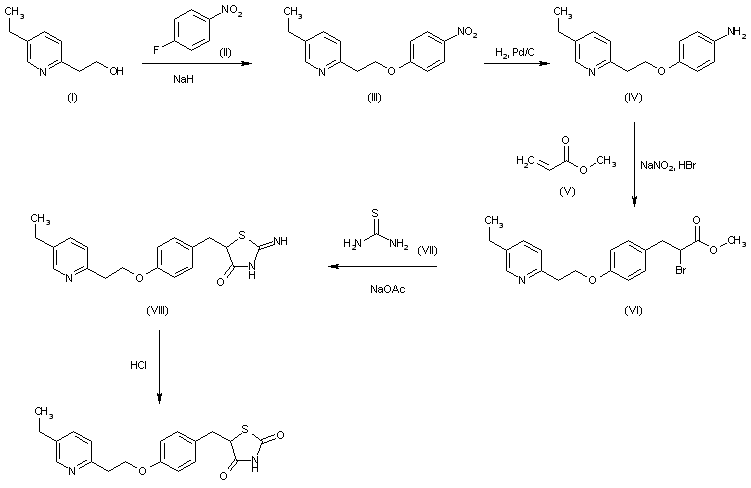
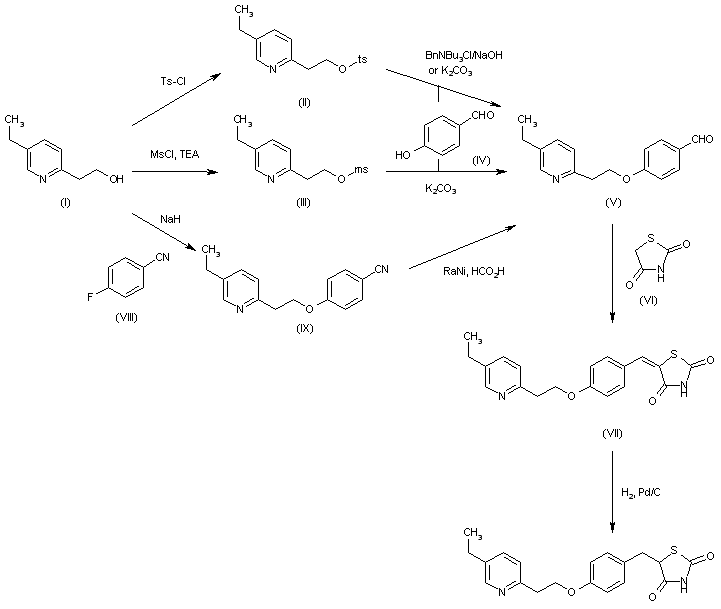
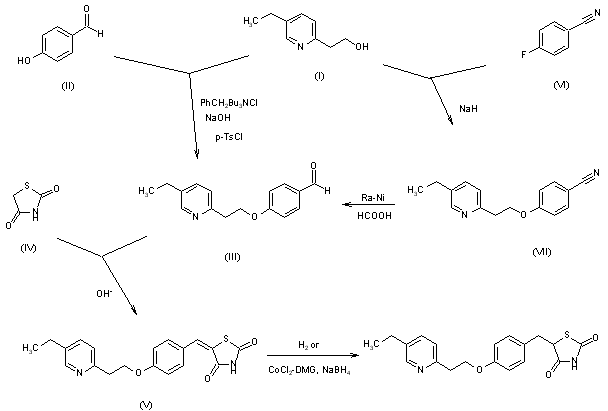
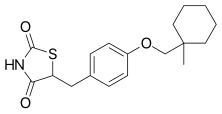
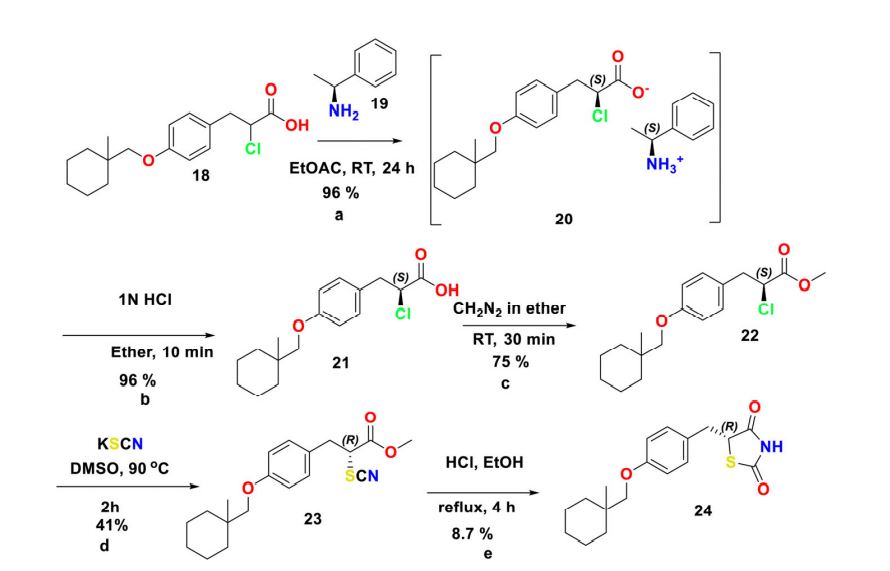
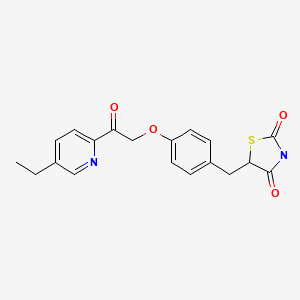 MITOGLITAZONE MSDC-0160; CAY 10415; 146062-49-9 5-[4-[2-(5-Ethylpyridin-2-yl)-2-oxoethoxy]benzyl]thiazolidine-2,4-dione 5-[[4-[2-(5-ethylpyridin-2-yl)-2-oxoethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione 5-(4-(2-(5-cthylpyridin-2-yl)- 2-oxoethoxy)benzyl)-1,3 -thiazolidiiie-2,4-dione Pfizer, INNOVATOR phase 2 MSD-9 MSDC-0160 PNU-91325 U-91325 BACKGROUND Thiazolidinedione analogs either prevent or ameliorate an insulin resistance state, which occurs genetically or is induced by dietary means. 5-(4-(2-(5-Ethylpyridin-2-yl)-2- oxoethoxy)benzyl)-l,3-thiazolidine-2,4-dione of Formula (I) (Mitoglitazonc) is an antidiabetic thiazolidinedione being evaluated for the treatment of non-insulin-dependent diabetes mellitus. Non-insulin-dependent diabetes mellitus (NIDDM) is a metabolic disease characterized by a reduction in the response of the peripheral target tissue to insulin and the inability of pancreatic insulin reserves to overcome the reduced response. Improvement of insulin sensitivity of the target tissue not only reduces the consequences of the disease but actually aids in the prevention of NIDDM. The synthesis of Mitoglitazone has been reported (J. Med. Chem. , 1996, 39, 5053- 5063; Org. Pro. Res. & Dev. (OPRD), 2002, 6, 721-728 and U.S. PaL No. 5,441,971). ..................................
MITOGLITAZONE MSDC-0160; CAY 10415; 146062-49-9 5-[4-[2-(5-Ethylpyridin-2-yl)-2-oxoethoxy]benzyl]thiazolidine-2,4-dione 5-[[4-[2-(5-ethylpyridin-2-yl)-2-oxoethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione 5-(4-(2-(5-cthylpyridin-2-yl)- 2-oxoethoxy)benzyl)-1,3 -thiazolidiiie-2,4-dione Pfizer, INNOVATOR phase 2 MSD-9 MSDC-0160 PNU-91325 U-91325 BACKGROUND Thiazolidinedione analogs either prevent or ameliorate an insulin resistance state, which occurs genetically or is induced by dietary means. 5-(4-(2-(5-Ethylpyridin-2-yl)-2- oxoethoxy)benzyl)-l,3-thiazolidine-2,4-dione of Formula (I) (Mitoglitazonc) is an antidiabetic thiazolidinedione being evaluated for the treatment of non-insulin-dependent diabetes mellitus. Non-insulin-dependent diabetes mellitus (NIDDM) is a metabolic disease characterized by a reduction in the response of the peripheral target tissue to insulin and the inability of pancreatic insulin reserves to overcome the reduced response. Improvement of insulin sensitivity of the target tissue not only reduces the consequences of the disease but actually aids in the prevention of NIDDM. The synthesis of Mitoglitazone has been reported (J. Med. Chem. , 1996, 39, 5053- 5063; Org. Pro. Res. & Dev. (OPRD), 2002, 6, 721-728 and U.S. PaL No. 5,441,971). .................................. 

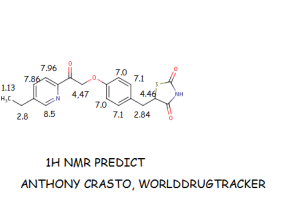
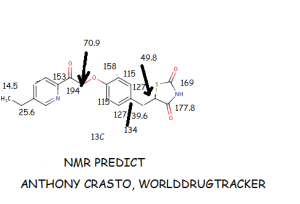
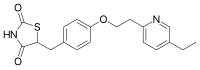

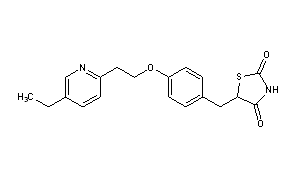








![[1860-5397-9-265-i12]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i12.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-i13]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i13.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-i14]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i14.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-i15]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i15.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-i16]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i16.png?max-width=550&background=EEEEEE)









 (I)
(I)









No comments:
Post a Comment