
| July-August 2012, Volume 2, No.4 |
 July-August 2012, Volume 2, No.4 July-August 2012, Volume 2, No.4Chemistry & Biology Interface, 2012, 2, 4, 206-257 (ISSN: 2249 – 4820) |
A Rapid microwave assisted synthesis of novel 1,4-dihydropyridines derivatives under aqueous medium
Shailesh Thakrar, Dhairya Bhavsar, Vicky Jain, Anamik Shah
| Chemistry & Biology Interface, 2012, 2, 4, 220-227 pg 220-227, Department of Chemistry, Saurashtra University, Rajkot-360005, India | ||
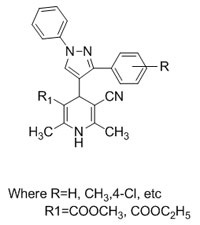
[Full Text-PDF]
Keywords: 1, 4-dihydro pyridines, Pyrazole aldehyde, One-pot, Microwave, Aqueous medium,
Fe+3 montmorillonite clay K-10, HY-zeolite.
Abstract: An environment friendly synthesis of 1,4-dihydropyridine derivatives was developed by one pot multi component reaction of pyrazole aldehyde, EAA/MAA, 3-amino crotononitrile and Fe+3 montmorillonite clay K-10/ HY-zeolite under microwave irradiation in aqueous medium. The structures of all synthesized compounds were well characterized by Mass, FT-IR, 1H NMR and elemental analysis.
Methyl 5-cyano-1,4-dihydro-2,6-dimethyl- 4-(1,3-diphenyl-1H-pyrazol-4-yl)pyridine- 3-carboxylate (5a): MP: 182-184 oC; IR (cm-1): 3489, 3367, 3198, 2974, 2897, 2332, 2260, 1707, 1660, 1587, 1519, 1435, 1356, 1282, 744, 688. MS: m/z = 426.17; 1H NMR (DMSO-d6) δ ppm: 2.14(s, 6H), 2.58(s, 3H), 4.91(s, 1H), 6.91-6.99(d, 2H), 7.20-7.22(t, 2H), 7.29-7.31(t, 1H), 7.45-7.49(t, 2H), 7.60-7.62(d, 1H), 7.71-7.73(d, 2H), 7.95(s, 1H), 8.74(s, 1H). MS: m/z: 410.17; Anal. Calcd. for C25H22N4O2: C, 73.15; H, 5.40; N,13.65; O,7.80; Found: C, 73.06; H, 5.36; N, 13.61; O,7.79(%).
//////////////








