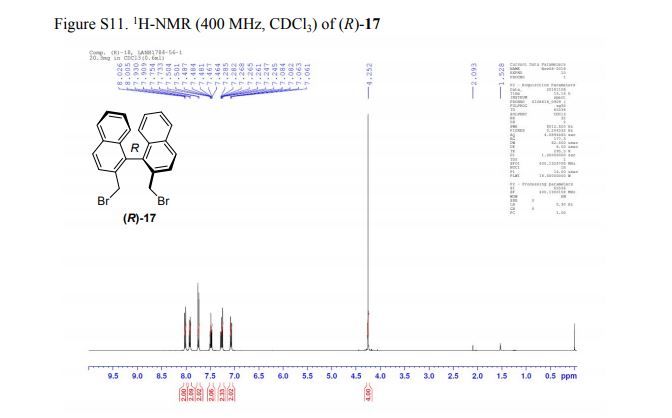
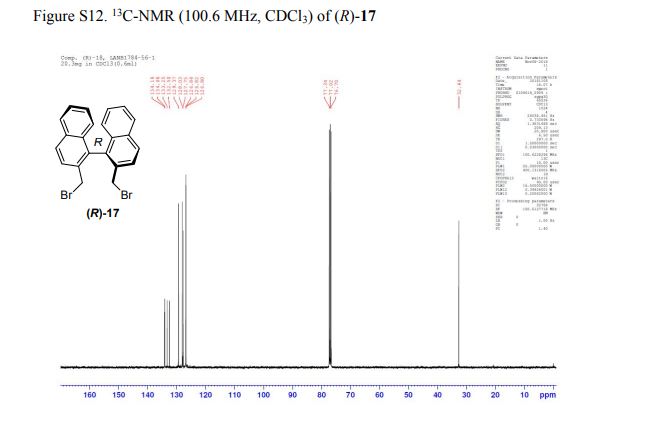
(R)-2,2′-bis(bromomethyl)-1,1′-binaphthalene ((R)-17) was prepared in the identical manner and had identical analytical properties to those given here.
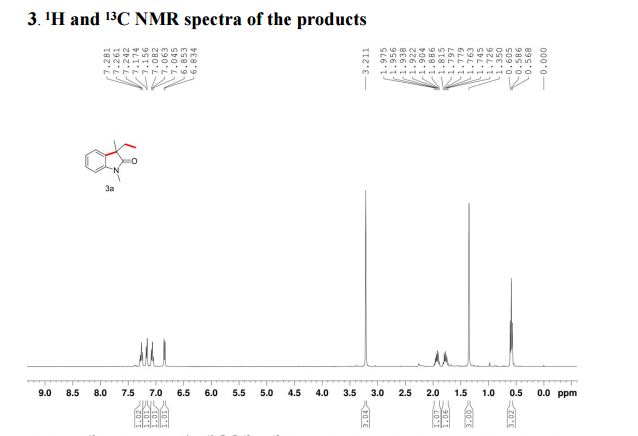
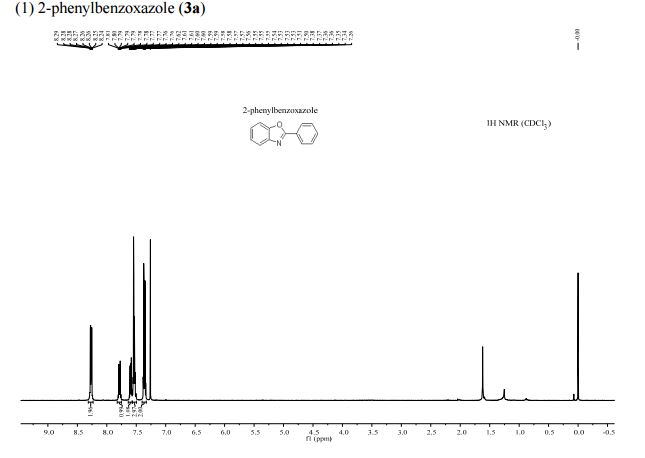
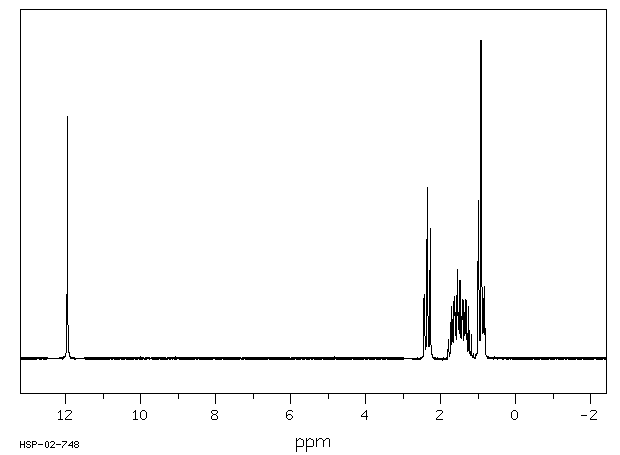
1H NMR (400 MHz, CDCl3): δ 4.25 (4H, s, 2 × CH2), 7.07 (2H, dd, J = 8.4, 0.8 Hz, ArH), 7.27 (2H, ddd, J = 8.4, 6.8, 1.2 Hz, ArH), 7.48 (2H, ddd, J = 8.2, 6.8, 1.2 Hz, ArH), 7.74 (2H, d, J = 8.6 Hz, ArH), 7.92 (2H, d, J = 8.2 Hz, ArH), 8.02 (2H, d, J = 8.6 Hz, ArH).
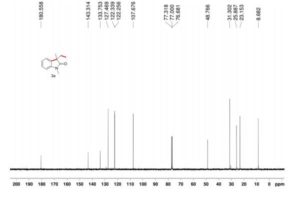
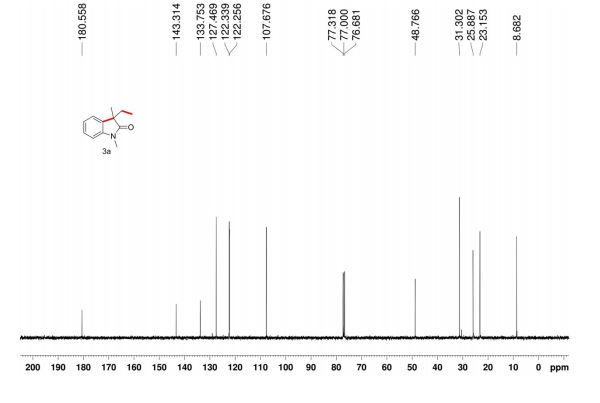
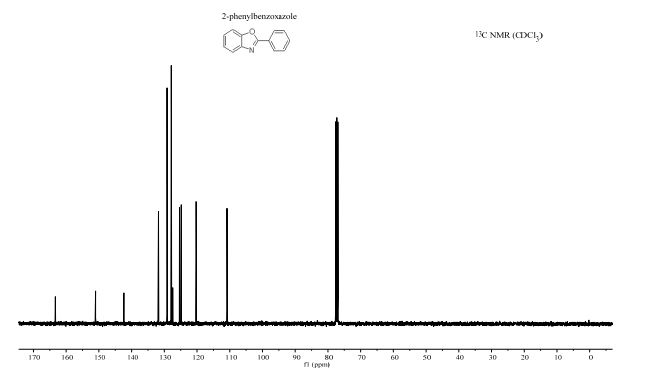
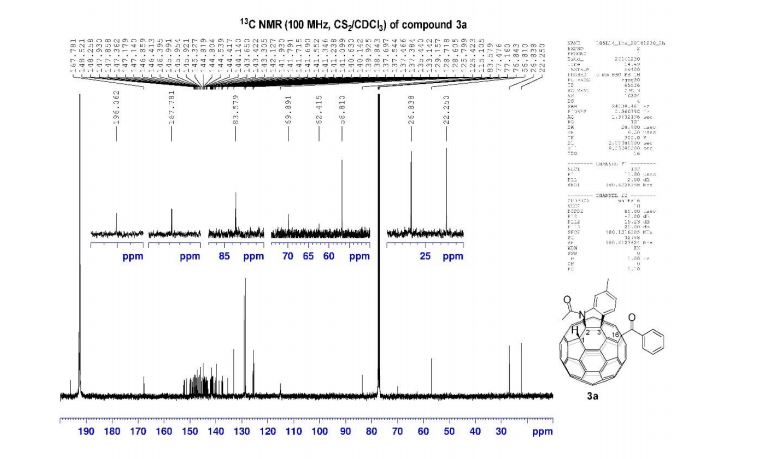
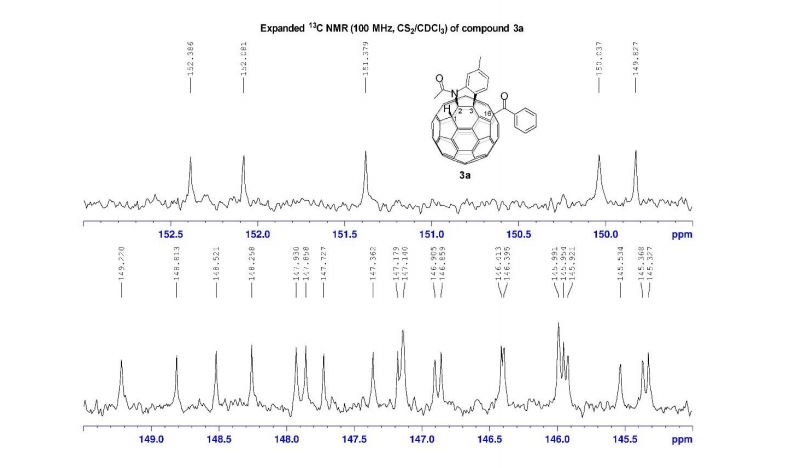
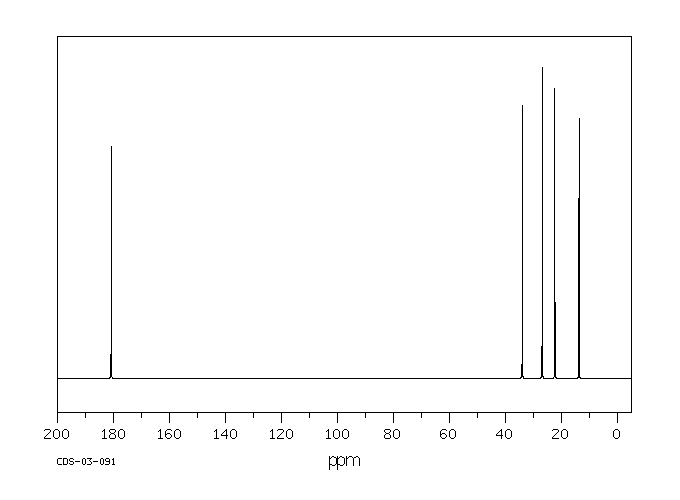
13C NMR (100.6 MHz, CDCl3): δ 32.6 (CH2), 126.80 (ArCH), 126.82 (ArCH), 126.84 (ArCH), 127.7 (ArCH), 128.0 (ArCH), 129.4 (ArCH), 132.5 (quaternary ArC), 133.3 (quaternary ArC), 134.1 (quaternary ArC), 134.2 (quaternary ArC).
[α]20D = +173.8° (c = 1.0, CHCl3).
An advanced process for large scale (500 g) preparation of a (3Z,5Z)-2,7-dihydro-1H-azepine-derived chiral tridentate ligand (Hamari ligand), widely used for asymmetric synthesis of tailor-made α-amino acids via the corresponding glycine Schiff base Ni(II) complex, is disclosed. The process includes amidation, bis-alkylation, and precipitation/purification of the target compound by TFA as a counterion.
Large Scale Synthesis of Chiral (3Z,5Z)-2,7-Dihydro-1H-azepine-Derived Hamari Ligand for General Asymmetric Synthesis of Tailor-Made Amino Acids
† Hamari Chemicals Ltd., 1-4-29 Kunijima, Higashi-Yodogawa-ku, Osaka 533-0024, Japan
‡ Hamari Chemicals USA, San Diego Research Center, 11494 Sorrento Valley Road, San Diego, California 92121, United States
§ Department of Organic Chemistry I, Faculty of Chemistry, University of the Basque Country UPV/EHU, Paseo Manuel Lardizábal 3, 20018 San Sebastián, Spain
∥ IKERBASQUE, Basque Foundation for Science, María Díaz de Haro 3, Plaza Bizkaia, 48013 Bilbao, Spain
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00406
Publication Date (Web): January 18, 2019
Copyright © 2019 American Chemical Society
*E-mail: motohiro-takahashi@hamari.co.jp., *E-mail: vadym.soloshonok@ehu.es.
This article is part of the Japanese Society for Process Chemistry special issue.
//////////////////












![2D [1H,1H]-TOCSY, 7.4 spectrum for Valeric acid](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000345/nmr/set01/spectra/HH_TOCSY.png)
![2D [1H,13C]-HSQC, 7.4 spectrum for Valeric acid](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000345/nmr/set01/spectra/1H_13C_HSQC.png)
![2D [1H,13C]-HMBC, 7.4 spectrum for Valeric acid](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000345/nmr/set01/spectra/1H_13C_HMBC.png)