HPLC-free Protein Synthesis

First methode for the fast chemical total synthesis of proteins without HPLC-purification
Read more
Organic Synthesis International by Dr Anthony Melvin Crasto Ph.D, Worlddrugtracker, Million hits on google on all sites, One lakh connections worldwide. Pushing boundaries.Interaction site for Organic chemists worldwide, Mail me at amcrasto@gmail.com if you like me




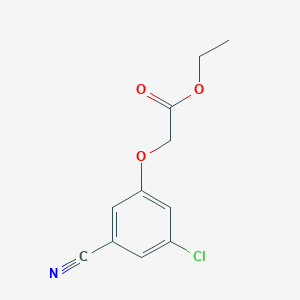
| Molecular Formula: | C11H10ClNO3 |
|---|---|
| Molecular Weight: | 239.655 g/mol |
 .
. Corresponding author email mh_1395@yahoo.com
Corresponding author email mh_1395@yahoo.com

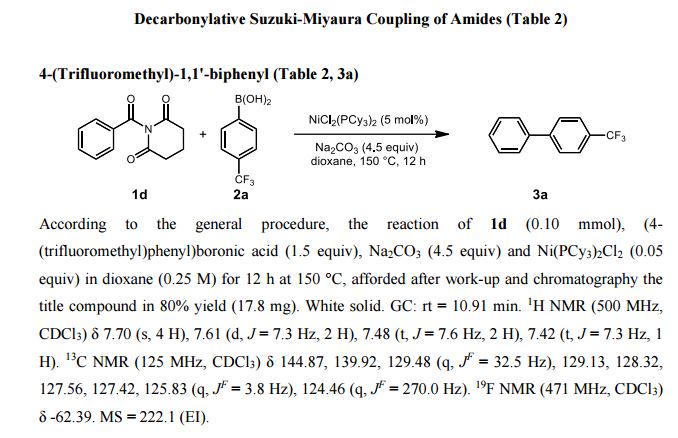
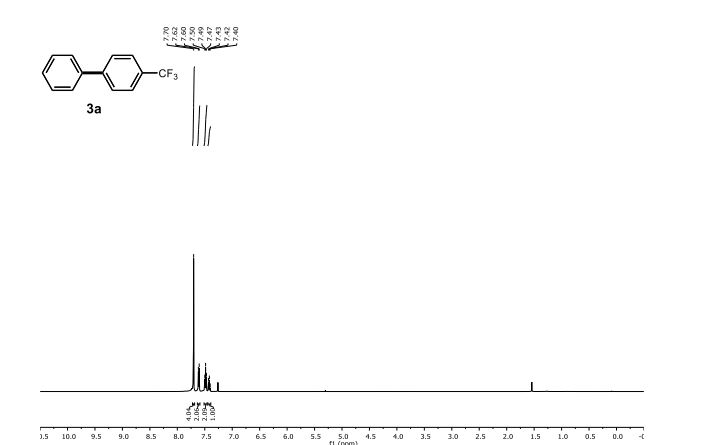
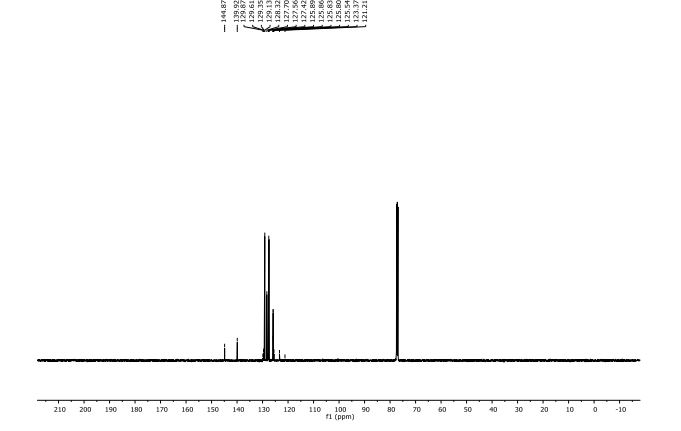
 | Michal Szostakemail: michal.szostak@rutgers.edu office: Olson 204
|

