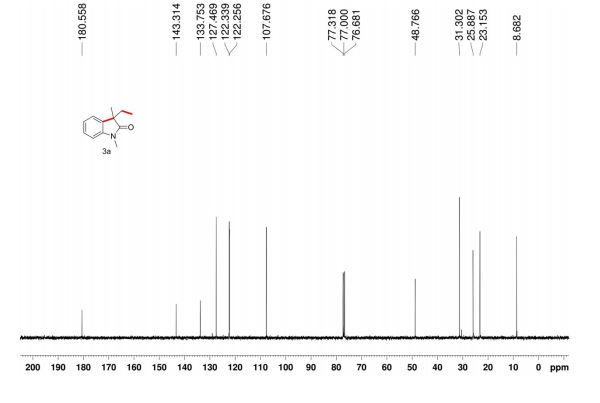
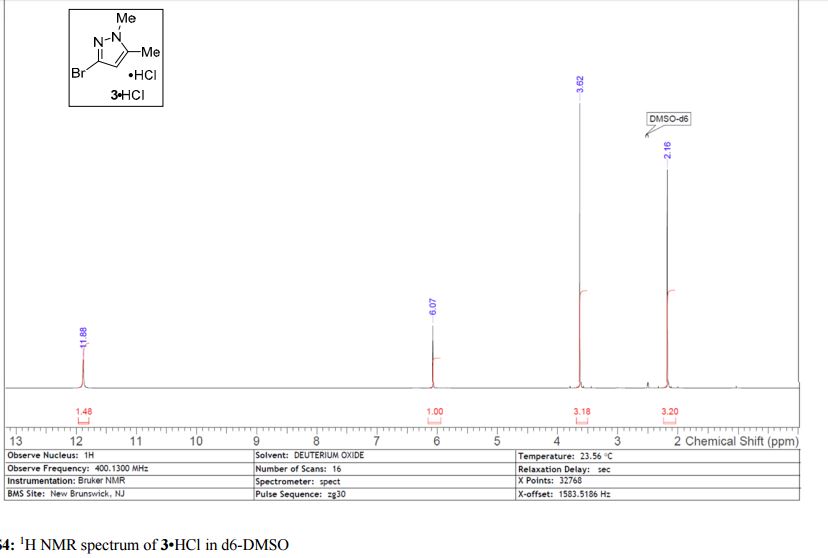
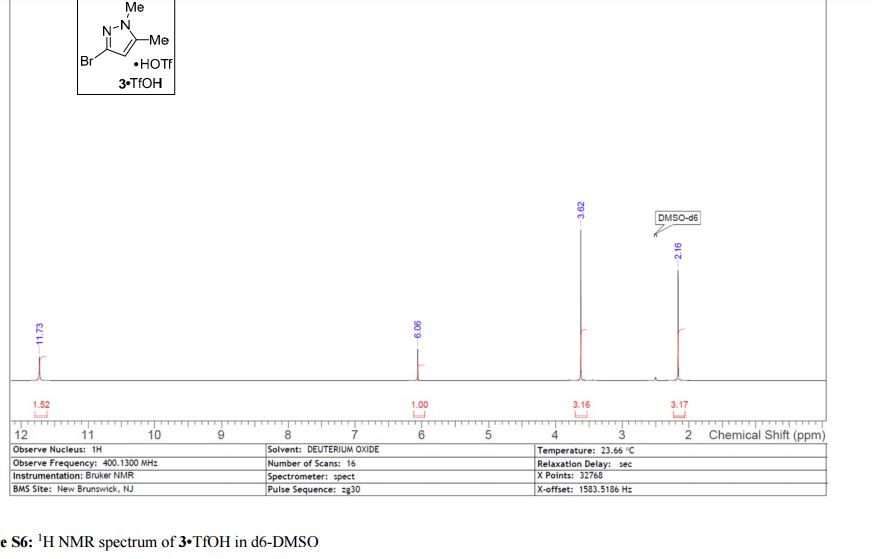
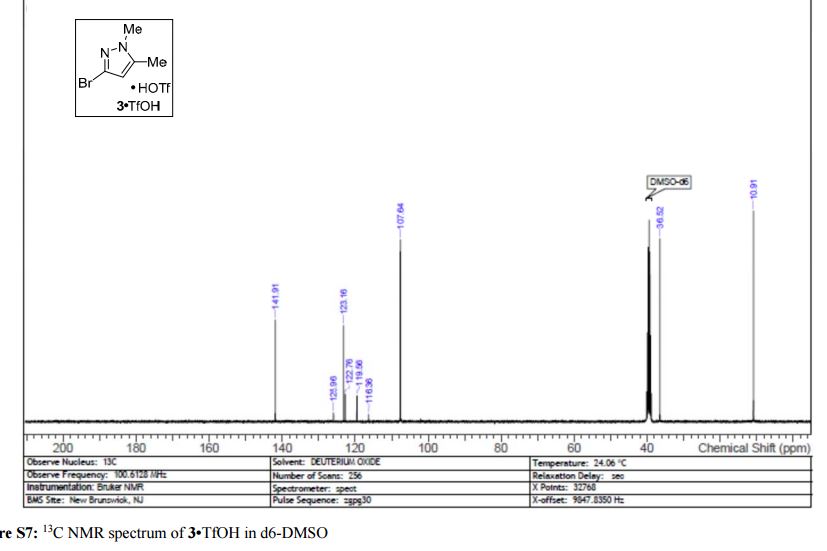
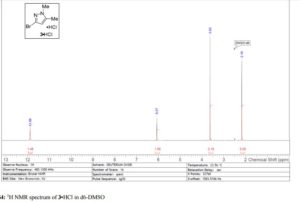
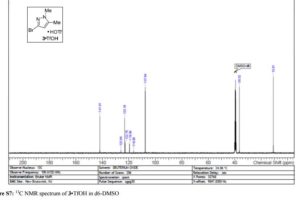
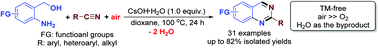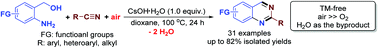Here is the another publication details
The paper is about m-CPBA/Chromium-VI mediated oxidation of oxazoles and published in Tetrahedron Letters recently.
m-Chloroperbenzoic
acid-oxchromium (VI)-mediated cleavage of
2,4,5-trisubstituted oxazoles
Pravin C. Patil and Frederick A. Luzzio*
Department of Chemistry,
University of Louisville, 2320 South Brook Street, Louisville, Kentucky 40292
USA
[Invited article: Tetrahedron Letters 2017, 58 (13),
1280-1282]
-------------------------------------------------------------------------------------------------------------------------------
Graphical Abstract:
Abstract:
An
array of 2-substituted-4,5-diphenyloxazoles were found to be cleaved to
triacylamines and diacylamines (imides) using a reagent system composed of
3-chloroperbenzoic acid (MCPBA) and 2,2'-bipyridinium chlorochromate (BPCC).
The 2-alkyl-4,5-diphenyloxazoles give imides (38-60%) as the predominant
cleavage product while the 2-aryl-4,5-diphenyloxazoles give triacylamines
(62-71%). Two mechanisms involving intermediates such as cyclic endoperoxides
or oxachromacycles were proposed. An application of the oxidative cleavage to
the multi-step synthesis of phoracantholide I seco acid is detailed.
--------------------------------------------------------------------------------------------------------------
Application
of methodology toward synthesis of Phoracantholide-I seco acid
Highlights
A new method for the cleavage of 2,4,5-trisubstituted oxazoles
to imides and triacylamines is detailed.
The oxidation system utilizes two reagents composed of a
peroxide and oxochromium (VI).
Mechanisms are proposed for the oxidative cleavage reaction.
A synthesis of (±)-phoracantholide seco-acid is detailed.
ABOUT GUEST BLOGGER
Dr. Pravin C. Patil
Postdoctoral Research Associate at University of Louisville
Dr. Pravin C Patil completed his B.Sc. (Chemistry) at ASC College Chopda (Jalgaon, Maharashtra, India) in 2001 and M.Sc. (Organic Chemistry) at SSVPS’S Science College Dhule in North Maharashtra University (Jalgaon, Maharashtra, India) in year 2003. After M.Sc. degree he was accepted for summer internship training program at Bhabha Atomic Research Center (BARC, Mumbai) in the laboratory of Prof. Subrata Chattopadhyay in Bio-organic Division. In 2003, Dr. Pravin joined to API Pharmaceutical bulk drug company, RPG Life Science (Navi Mumbai, Maharashtra, India) and worked there for two years. In 2005, he enrolled into Ph.D. (Chemistry) program at Institute of Chemical Technology (ICT), Matunga, Mumbai, aharashtra, under the supervision of Prof. K. G. Akamanchi in the department of Pharmaceutical Sciences and Technology.
After finishing Ph.D. in 2010, he joined to Pune (Maharashtra, India) based pharmaceutical industry, Lupin Research Park (LRP) in the department of process development. After spending two years at Lupin as a Research Scientist, he got an opportunity in June 2012 to pursue Postdoctoral studies at Hope College, Holland, MI, USA under the supervision of Prof. Moses Lee. During year 2012-13 he worked on total synthesis of achiral anticancer molecules Duocarmycin and its analogs. In 2014, he joined to Prof. Frederick Luzzio at the Department for Chemistry, University of Louisville, Louisville, KY, USA to pursue postdoctoral studies on NIH sponsored project “ Structure based design and synthesis of Peptidomimetics targeting P. gingivalis.
During his research experience, he has authored 23 international publications in peer reviewed journals and inventor for 4 patents.
//////////////guest blogger, pravin patil